Life @ NewLife
- Home
- /
- Life @ New Life


Culture & Celebrations
NewLife fosters Respect, Equality, and Inclusivity within its employees, stakeholders, suppliers, and vendors. Our diversity plan recruits the best talent regardless of gender, age, race, disabilities, and nationality. This facilitates a diverse work culture where people with different skill sets come together to create an environment of creativity and mutual respect. Women represent half of our workforce.
We endeavor to create a sustainable value system for people, communities, partners, and our planet.We have established a compliance team that guides employees in following ethical business practices while incorporating integrity and honesty in their behavior.
NewLife Medicals encourages employees to make their lives productive while enjoying the workplace. We encourage them to bring their own goals and achieve them with us with our employee-friendly work policies, support system, safe environment, and growth opportunities. Celebrating life in every form is a core of every NewLife Medicals employees existence.




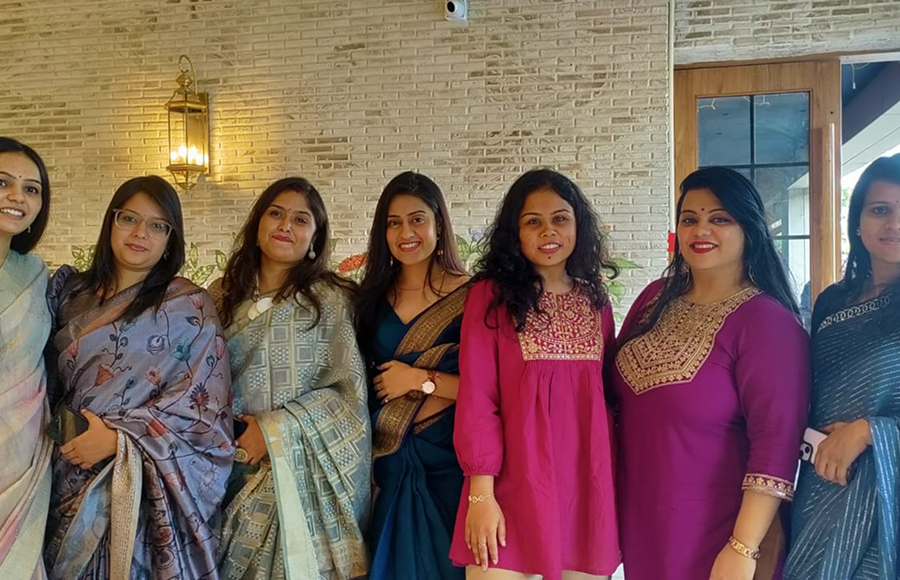
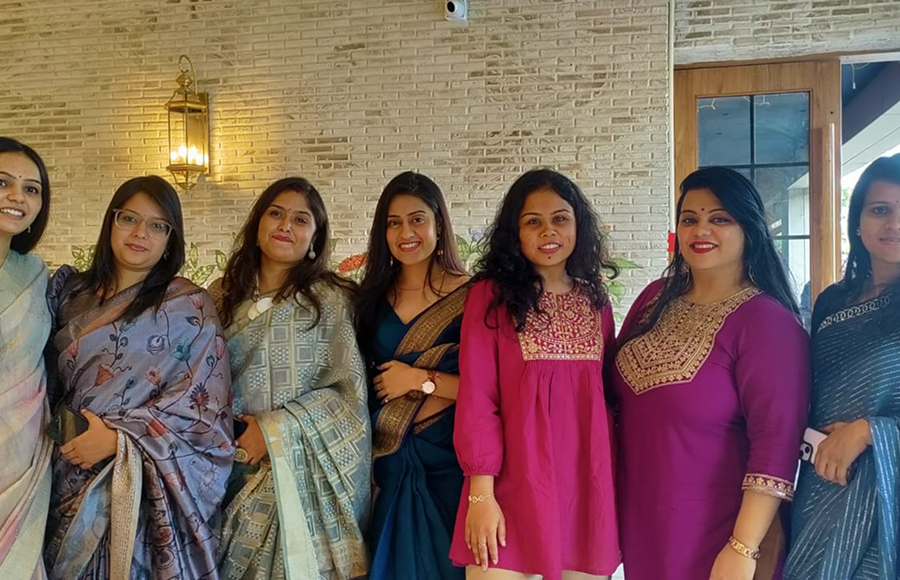






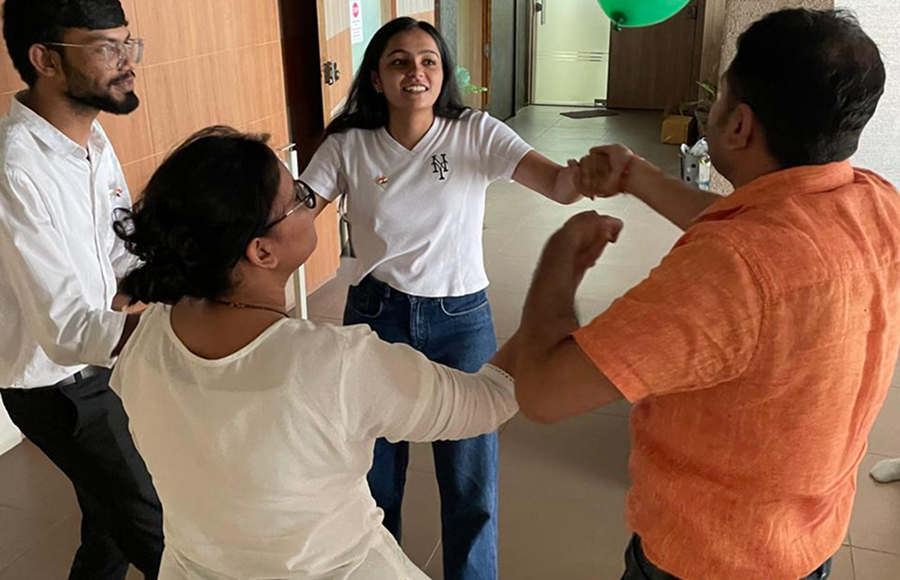
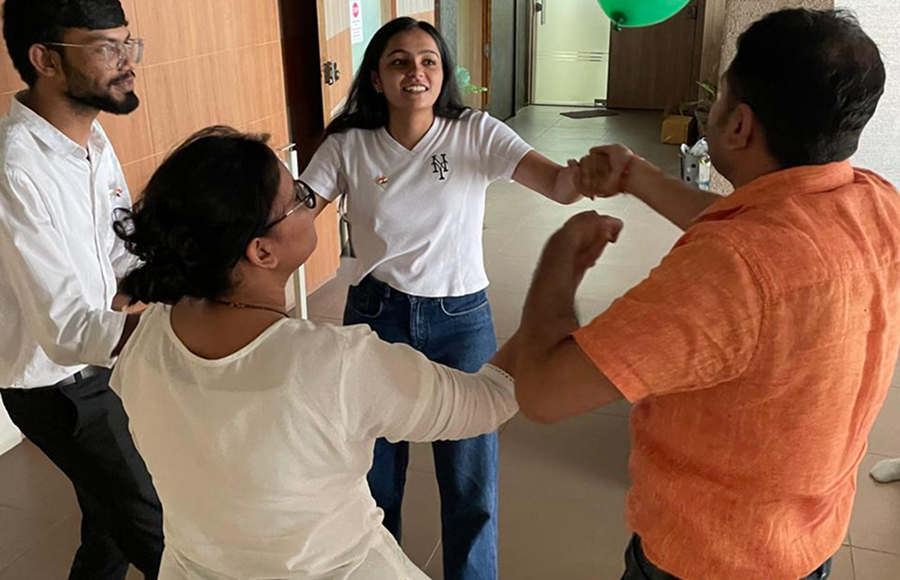




































Celebration






































Product Launch Events


















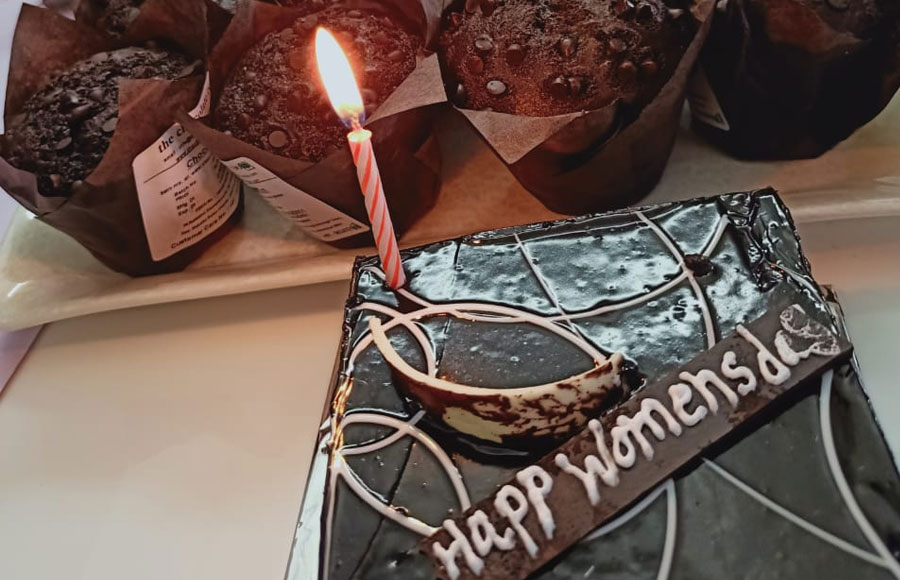
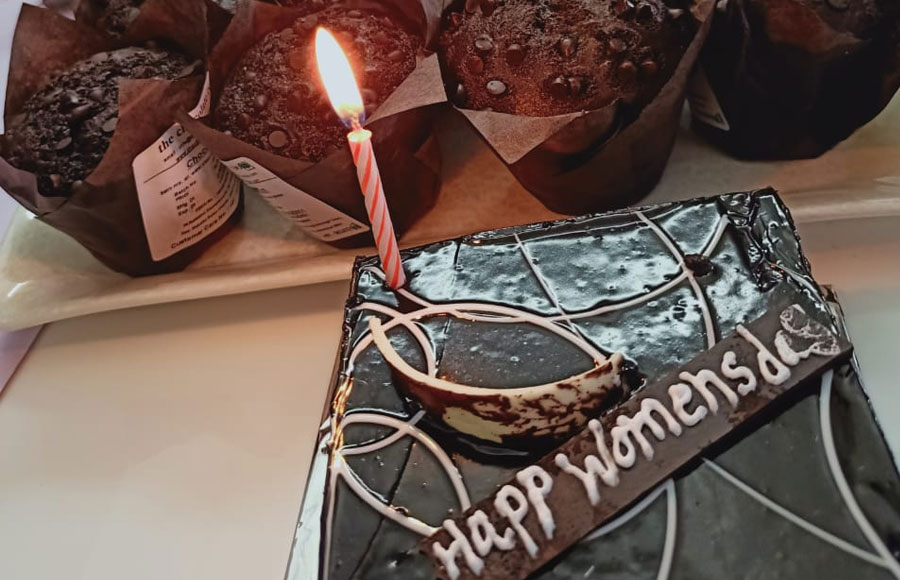








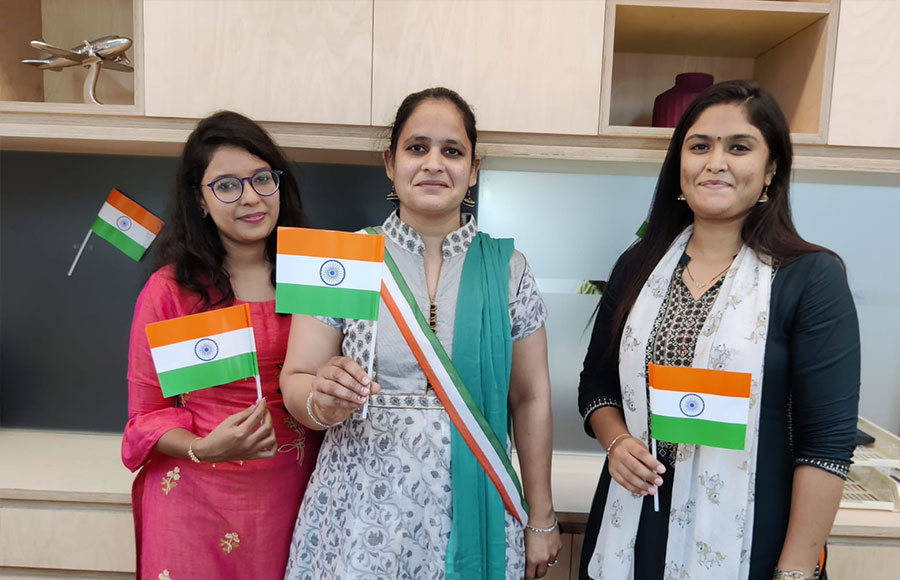
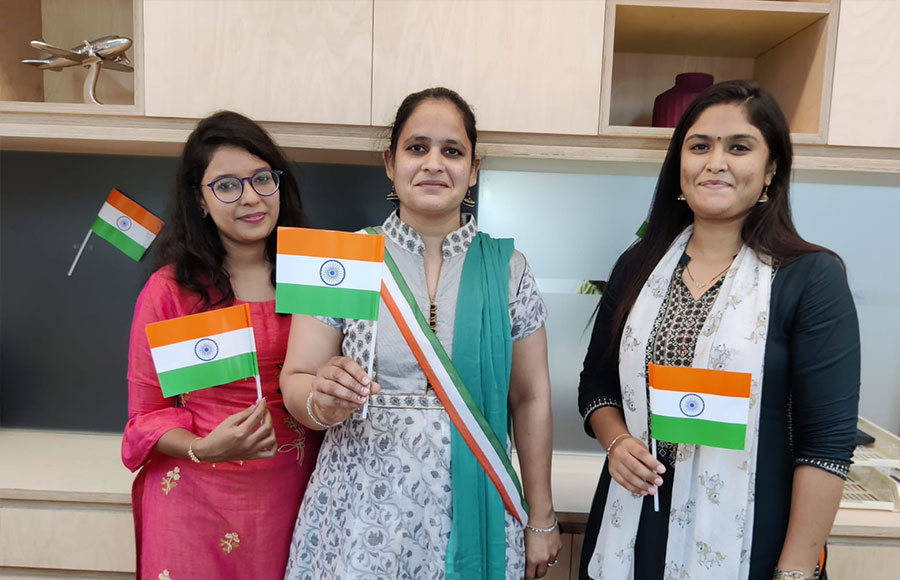




Celebration






























Product Launch Events
NewLife @ Campus
We at NLM are inspired by our purpose of ‘Being the Trusted Lifeline’ and strive to extend this philosophy to our aspirants and their families. Our endeavour is to build a high-performance and meritocratic work culture with Trust as the cornerstone.
Our Campus Program is focused on building superior talent pipeline from reputed management and pharma institutes in the country. This endeavour therefore helps us to build entry-level talent pipeline.






































Open positions
Job Summary :
This position will be responsible for growing NewLife Medicals business in its assigned region within the China. Strategically developing and executing a growth-centric account plan, with a strong understanding of market demand and fluctuations and the ability to provide our customers with products they need; ultimately growing the Business. Portfolio of service offerings within the clinical trial supplies or comparator drug supplies marketplace. This is a revenue quota-carrying sales position. This is a remote, field-based role that may require travel to build/support a client base.
Essential Duties and Responsibilities :
- Have a working knowledge of Branded pharmaceutical market, contracting, clinical trial supplies or comparator drug supplies, distribution/wholesaler channels, pricing structures, and clinical trial site
- Pursuit of net new accounts within the China.
- Achieve/Outperform assigned annual sales and profitability targets.
- Develop and manage sales pipeline, prospect, and assess sales and move multiple transactions simultaneously through the sales pipeline.
- Manage and track customer interaction and transactional information in ERP SAP.
- Coordinate pre-sales resources throughout the sales cycle.
- Execute a land-and-expand sales strategy with well-thought-out enterprise sales strategies.
- Develop strong and ongoing relationships with Key Decision Makers, Key Influencers, Technical Buyers, and Economic Buyers in target accounts.
- Provide regular reporting of prospecting, pipeline, and forecast through the ERP SAP CRM module.
- Keep abreast of competition, competitive issues etc.
- Practice effective, excellent communication with management, customers, and support staff.
- Resolves customer complaints by investigating service problems, developing solutions, preparing reports, and making recommendations to management.
- Maintains professional and technical knowledge by attending medical exhibitions, reviewing professional publications, establishing personal networks, and participating in professional societies.
- Attend and participate into the events or exhibitions to generate the new business customers and suppliers also build the business relationships with the clients.
- Must read industry journals and stay abreast of changes in the field, including current state and federal legislation about pharmaceutical sales.
Work Experience Qualifications :
- Proven 5 to 12+ years of work experience as a Pharmaceutical Salesperson, preferably in the clinical trial supplies, pharmaceuticals outsourcing team, comparator drug sourcing and supplies.
- Result driven orientation having solid customer service attitude with excellent negotiation skills.
- Previous experience in SAP & MS Office will be an added advantage
- Must have keen attention to detail and possess proper phone and email etiquette.
- Ability to stay organized while effectively prioritizing multiple projects at once.
- High attention to detail when completing projects.
- Person should be in position to travel for meetings and exhibitions and events.
- Strong time management skills to complete projects by deadlines.
- Self-motivated individual who takes ownership of their projects.
- Administrative skills (MS Office and Google Workspace are required).
- Maintain excellent verbal, writing, and language skills.
Education Qualifications :
- Masters’s Degree in health sciences, marketing, or related field of Pharmaceuticals.
Why You Should Join NewLife Medicals
- Join a high growth and fast paced global organization with a people focused culture
- Global exposure, defined career path and annual performance review and feedback process
NewLife Medicals fosters Respect, Equality, and Inclusivity within its employees, stakeholders, suppliers, and vendors. Our diversity plan recruits the best talent regardless of gender, age, race, disabilities, and nationality. This facilitates a diverse work culture where people with different skill sets come together to create an environment of creativity and mutual respect. Women represent half of our workforce.
We endeavor to create a sustainable value system for people, communities, partners, and our planet. We have established a compliance team that guides employees in following ethical business practices while incorporating integrity and honesty in their behavior.
NewLife Medicals encourages employees to make their lives productive while enjoying the workplace. We encourage them to bring their own goals and achieve them with us with our employee-friendly work policies, support system, safe environment, and growth opportunities. Celebrating life in every form is a core of every NewLife Medicals employee’s existence.
Job Summary :
This position will be responsible for growing NewLife Biopharma Limited. business in its assigned region within the Europe. Strategically developing and executing a growth-centric account plan, with a strong understanding of market demand and fluctuations and the ability to provide our customers with products they need; ultimately growing the NewLife Biopharma Limited. Portfolio of service offerings within the clinical trial supplies or comparator drug supplies marketplace. This is a revenue quota-carrying sales position. This is a remote, field-based role that may require travel to build/support a client base.
Essential Duties and Responsibilities :
- Have a working knowledge of Branded pharmaceutical market, contracting, clinical trial supplies or comparator drug supplies, distribution/wholesaler channels, pricing structures, and clinical trial sites.
- Pursuit of net new accounts within the Europe.
- Achieve/Outperform assigned annual sales and profitability targets.
- Develop and manage sales pipeline, prospect, and assess sales and move multiple transactions simultaneously through the sales pipeline.
- Manage and track customer interaction and transactional information in ERP SAP.
- Coordinate pre-sales resources throughout the sales cycle.
- Execute a land-and-expand sales strategy with well-thought-out enterprise sales strategies.
- Develop strong and ongoing relationships with Key Decision Makers, Key Influencers, Technical Buyers, and Economic Buyers in target accounts.
- Provide regular reporting of prospecting, pipeline, and forecast through the ERP SAP CRM module.
- Keep abreast of competition, competitive issues etc.
- Practice effective, excellent communication with management, customers, and support staff.
- Resolves customer complaints by investigating service problems, developing solutions, preparing reports, and making recommendations to management.
- Maintains professional and technical knowledge by attending medical exhibitions, reviewing professional publications, establishing personal networks, and participating in professional societies.
- Represent in various exhibitions of clinical trial space and identify new partners for the business and build the business relationships with those accounts.
- Must read industry journals and stay abreast of changes in the field, including current state and federal legislation about pharmaceutical sales.
Work Experience Qualifications :
- Proven 5 to 12+ years of work experience as a Pharmaceutical Salesperson, preferably in the clinical trial supplies, pharmaceuticals outsourcing team, comparator drug sourcing and supplies.
- Result driven orientation having solid customer service attitude with excellent negotiation skills.
- Previous experience in SAP & MS Office will be an added advantage
- Must have keen attention to detail and possess proper phone and email etiquette.
- Ability to stay organized while effectively prioritizing multiple projects at once.
- High attention to detail when completing projects.
- Person should be in position to travel for meetings and exhibitions and events.
- Strong time management skills to complete projects by deadlines.
- Self-motivated individual who takes ownership of their projects.
- Administrative skills (MS Office and Google Workspace are required).
- Maintain excellent verbal, writing, and language skills.
Education Qualifications :
- Master’s Degree in health sciences, marketing, or related field of Pharmaceuticals.
Why You Should Join NewLife Medicals
- Join a high growth and fast paced global organization with a people focused culture
- Global exposure, defined career path and annual performance review and feedback process
NewLife Medicals fosters Respect, Equality, and Inclusivity within its employees, stakeholders, suppliers, and vendors. Our diversity plan recruits the best talent regardless of gender, age, race, disabilities, and nationality. This facilitates a diverse work culture where people with different skill sets come together to create an environment of creativity and mutual respect. Women represent half of our workforce.
We endeavor to create a sustainable value system for people, communities, partners, and our planet. We have established a compliance team that guides employees in following ethical business practices while incorporating integrity and honesty in their behavior.
NewLife Medicals encourages employees to make their lives productive while enjoying the workplace. We encourage them to bring their own goals and achieve them with us with our employee-friendly work policies, support system, safe environment, and growth opportunities. Celebrating life in every form is a core of every NewLife Medicals employee’s existence.
This position will be responsible for growing NewLife Medicals (USA) Inc. business in its assigned region within the United States. Strategically developing and executing a growth-centric account plan, with a strong understanding of market demand and fluctuations and the ability to provide our customers with products they need; ultimately growing the NewLife Medicals (USA) Inc. Portfolio of service offerings within the clinical trial supplies or comparator drug supplies marketplace. This is a revenue quota-carrying sales position. This is a remote, field-based role that may require travel to build/support a client base.
Essential Duties and Responsibilities :- Have a working knowledge of Branded pharmaceutical market, contracting, clinical trial supplies or comparator drug supplies, distribution/wholesaler channels, pricing structures, and clinical trial sites.
- Pursuit of net new accounts within the USA.
- Achieve/Outperform assigned annual sales and profitability targets.
- Develop and manage sales pipeline, prospect, and assess sales and move multiple transactions simultaneously through the sales pipeline.
- Manage and track customer interaction and transactional information in ERP SAP.
- Coordinate pre-sales resources throughout the sales cycle.
- Execute a land-and-expand sales strategy with well-thought-out enterprise sales strategies.
- Develop strong and ongoing relationships with Key Decision Makers, Key Influencers, Technical Buyers, and Economic Buyers in target accounts.
- Provide regular reporting of prospecting, pipeline, and forecast through the ERP SAP CRM module.
- Keep abreast of competition, competitive issues etc.
- Practice effective, excellent communication with management, customers, and support staff.
- Resolves customer complaints by investigating service problems, developing solutions, preparing reports, and making recommendations to management.
- Maintains professional and technical knowledge by attending medical exhibitions, reviewing professional publications, establishing personal networks, and participating in professional societies.
- Attend and participate into the events or exhibitions to generate the new business customers and suppliers also build the business relationships with the clients.
- Must read industry journals and stay abreast of changes in the field, including current state and federal legislation about pharmaceutical sales.
- Proven 5-12+ years of work experience as a Pharmaceutical Salesperson, preferably in the clinical trial supplies, pharmaceuticals outsourcing team, comparator drug sourcing and supplies.
- Result driven orientation having solid customer service attitude with excellent negotiation skills.
- Previous experience in SAP & MS Office will be an added advantage
- Must have keen attention to detail and possess proper phone and email etiquette.
- Ability to stay organized while effectively prioritizing multiple projects at once.
- High attention to detail when completing projects.
- Person should be in position to travel for meetings and exhibitions and events.
- Strong time management skills to complete projects by deadlines.
- Self-motivated individual who takes ownership of their projects.
- Administrative skills (MS Office and Google Workspace are required).
- Maintain excellent verbal, writing, and language skills.
- Masters’s Degree in health sciences, marketing, or related field of Pharmaceuticals.
- Join a high growth and fast paced global organization with a people focused culture
- Global exposure, defined career path and annual performance review and feedback process
NewLife Medicals fosters Respect, Equality, and Inclusivity within its employees, stakeholders, suppliers, and vendors. Our diversity plan recruits the best talent regardless of gender, age, race, disabilities, and nationality. This facilitates a diverse work culture where people with different skill sets come together to create an environment of creativity and mutual respect. Women represent half of our workforce.
We endeavor to create a sustainable value system for people, communities, partners, and our planet. We have established a compliance team that guides employees in following ethical business practices while incorporating integrity and honesty in their behavior.
NewLife Medicals encourages employees to make their lives productive while enjoying the workplace. We encourage them to bring their own goals and achieve them with us with our employee-friendly work policies, support system, safe environment, and growth opportunities. Celebrating life in every form is a core of every NewLife Medicals employee’s existence.
This position for Quality Management Systems (QMS) will ensure compliance with Good Distribution Practices (GDP), 21 CFR regulations, and other applicable standards in a warehouse environment specializing in clinical trial supplies. This role involves implementing, monitoring, and enhancing quality systems to maintain regulatory compliance and ensure operational excellence.
Essential Duties and Responsibilities :- Manage and oversee the QMS processes, including documentation, control, and implementation of policies and procedures in compliance with GDP, 21 CFR, and industry standards.
- Conduct routine internal & external audits of warehouse operations to ensure adherence to quality guidelines, regulatory requirements, and standard operating procedures (SOPs).
- Coordinate and manage deviation investigations, CAPA (Corrective and Preventive Actions), change control processes and Risk Assessments.
- Ensure Vendor/Customer Verification & Qualification on timely basis also should do audit if required on site of partners site.
- Accurate documentation check for the each transaction been carried out for the inbound and outbound by the operation team.
- Ensure accuracy and effective maintenance of the eQMS portal for all quality-related documentation and activities.
- Reviewing and Authorizing Quality related documents and agreements.
- Prepare and review quality documentation such as SOPs using eQMS portal, validation protocols, and training records to ensure they meet regulatory and organizational standards.
- Support regulatory inspections, internal audits, and customer audits by providing necessary documentation and ensuring compliance readiness.
- Monitor and evaluate the effectiveness of QMS processes through metrics and reporting, identifying areas for improvement.
- Conduct training sessions for staff on QMS policies, GDP requirements, and regulatory compliance.
- Collaborate with cross-functional teams, including warehouse operations, logistics, and regulatory affairs, to address quality-related issues and implement improvements.
- Training the operation team personnel in warehouse.
- Stay updated on evolving regulatory requirements and incorporate changes into QMS practices.
- Yearly review on the SOPs if any changes need to be made and upgrade the processes.
- All documents record should be kept in controlled and secured manner.
- Proven 5+ years of work experience in QMS role within the Pharmaceutical Industry.
- Result driven orientation having solid customer service attitude with excellent negotiation skills.
- Previous experience in SAP & MS Office will be an added advantage
- Must have keen attention to detail and possess proper phone and email etiquette.
- Ability to stay organized while effectively prioritizing multiple projects at once.
- High attention to detail when completing projects.
- Person should be in position to travel for meetings and exhibitions and events.
- Strong time management skills to complete projects by deadlines.
- Self-motivated individual who takes ownership of their projects.
- Administrative skills (MS Office and Google Workspace are required).
- Maintain excellent verbal, writing, and language skills.
- Masters’s Degree in health sciences, or related field of Pharmaceuticals.
- Join a high growth and fast paced global organization with a people focused culture
- Global exposure, defined career path and annual performance review and feedback process
NewLife Medicals fosters Respect, Equality, and Inclusivity within its employees, stakeholders, suppliers, and vendors. Our diversity plan recruits the best talent regardless of gender, age, race, disabilities, and nationality. This facilitates a diverse work culture where people with different skill sets come together to create an environment of creativity and mutual respect. Women represent half of our workforce.
We endeavor to create a sustainable value system for people, communities, partners, and our planet. We have established a compliance team that guides employees in following ethical business practices while incorporating integrity and honesty in their behavior.
NewLife Medicals encourages employees to make their lives productive while enjoying the workplace. We encourage them to bring their own goals and achieve them with us with our employee-friendly work policies, support system, safe environment, and growth opportunities. Celebrating life in every form is a core of every NewLife Medicals employee’s existence.
Job description:
- B2B Business Development of Pharmaceutical formulation.
Meeting Purchase / Procurement / BD team in - Pharmaceutical Company / R&D/ Sponsor companies for information on new projects in pipeline &understanding current / future needs wrt RLD’s / Comparator Product
- Meet potential clients by growing, maintaining, and leveraging network.
- Identify potential clients, and the decision makers within the client organization.
- Research and build relationships with new clients.
- Work with team to develop proposals that speak to the clients needs, concerns, and objectives.
- Present new products and services and enhance existing relationships.
- Work with technical staff and other internal colleagues to meet customer needs.
- Arrange and participate in internal and external client debriefs.
- Attend industry functions, such as exhibitions and conferences, and provide feedback and information on market and creative trends.
- Present to and consult with mid and senior level management on business trends with a view to developing new services.
- Identify opportunities for campaigns, servicesthat will lead to an increase in sales.
- Using knowledge of the market and competitors, identify and develop the company’s unique selling propositions and differentiators.
Requirement :
- BSc / BPharm & MBA with 5+ years’ experience in pharmaceutical sales / marketing / business development
- Must be self-motivating, organized and proactive
- Strong technical aptitude including demonstrated experience in applying scientific reasoning to solve complex problems.
- Ability to work hours necessary to support completion of work
Open positions
Job description:
- Dossier creation, compilation, review and submission.
- Develop and communicate regulatory strategy for global markets and manage deliverables.
- Responding to deficiency letter and technical queries from regulatory agencies.
- Maintain working knowledge of relevant guidelines to ensure regulatory compliance of major Global markets ie US, Europe, GCC, SEA etc.
- Coordinate with internal departments and timely reminder for arranging documents pertaining to CDSCO applications
- Filling data and submission of proforma of CDEC for form 12 A under Named Patient Drug Supplies.
- To coordinate and maintain record of day to day requirement of technical documents.
- To work on arrangement of Registration samples in accordance with the production and QA
departments for Exports Division. - To coordinate with Clients, Drug Registration Agencies etc for product registration related compliance need &legalization of product registration documents
- Working closely with cross functional teams within the organization and third party teams.
- Maintain good collaboration with all parties for the ongoing projects
Requirement :
- B. Pharm / M. Pharm with experience of >3 years in pharmaceutical regulatory dept.
Job description:
- B2B Business Development of Pharmaceutical formulation.
Meeting Purchase / Procurement / BD team in - Pharmaceutical Company / R&D/ Sponsor companies for information on new projects in pipeline &understanding current / future needs wrt RLD’s / Comparator Product
- Meet potential clients by growing, maintaining, and leveraging network.
- Identify potential clients, and the decision makers within the client organization.
- Research and build relationships with new clients.
- Work with team to develop proposals that speak to the clients needs, concerns, and objectives.
- Present new products and services and enhance existing relationships.
- Work with technical staff and other internal colleagues to meet customer needs.
- Arrange and participate in internal and external client debriefs.
- Attend industry functions, such as exhibitions and conferences, and provide feedback and information on market and creative trends.
- Present to and consult with mid and senior level management on business trends with a view to developing new services.
- Identify opportunities for campaigns, servicesthat will lead to an increase in sales.
- Using knowledge of the market and competitors, identify and develop the company’s unique selling propositions and differentiators.
Requirement :
- BSc / BPharm & MBA with 5+ years’ experience in pharmaceutical sales / marketing / business development
- Must be self-motivating, organized and proactive
- Strong technical aptitude including demonstrated experience in applying scientific reasoning to solve complex problems.
- Ability to work hours necessary to support completion of work
Job description:
- Coordinate and monitors inbound shipments and clearance activity in timely manner through CHA to avoid penalty for late filling and demurrage.
- Maintaining an import / export tracker and track the shipment until the shipment reach the destination
- Support for On Time Delivery to the customer
- Co-ordination with freight forwarders as per the customer requirements
- Preparation of outbound shipping documentation in accordance with already laid procedures
- Provide provision statement for import/export shipments to Finance
- Good communication and interpersonal skills
- Responsible for implementing Corrective and Preventive actions
- Maintaining the original shipping documents
- Support in audit preparation for Logistics team
- Up-to-date knowledge about Customs related to Imports and exports
- Report’s status of activities to the Manager
- Flexible to take up new challenges &performs other related duties as required.
- Willing to work in US Shifts timings
Responsibilities:
- Cold Chain management and expertise – Supports the execution of temperature-controlled shipments by liaising / coordinating with stakeholdersie with supplier, warehouse , Freight Forwarders , CHA etc
- Lead efforts for outbound / inbound product packaging validation
- Develop domestic and international solutions of shipping cold chain materials, to include the coordination of import clearance review with clearance brokers
- Accountable for ensuring adherence to strict regulatory requirements to ensure product quality.
- Works with all internal teams and ensure delivery standards by effective communication.
- Assists and coordinates review of all shipping protocols, reports, records, and procedures to assure compliance and completeness.
- Support activities related to any cold chain preparation, shipping or packaging validation efforts
- Develops the materials space requirement plan for cold storage chambers and assets.
- Works with Finance on end of month inventory reconciliation
- Organizes material according to set standards regarding order, clarity, conciseness, style, and terminology
- Maintains records and files of work and revisions
- Participates in the Inventory Control Programs.
- Verifies inventory computations by coordinating with warehouse staff and comparing them to physical counts of stock, and investigate discrepancies or adjust error
- Subject Matter expert within the logistical space, including freight forwarding air, land transportation, customs brokerage, clinical supplies, and parcels.
- Develop the management process to ensure timely deliveries (Inbound & Outbound) and in compliance with all import/export regulations including country of origin
- Manage global/regional freight forwarder and 3PLs negotiation, contracts, quality impact, service, and relationships.
- Establish necessary protocols, SOPs, Work Practices related to logistics.
- Provide leadership and support to the implementation of new systems, processes, identifying appropriate vendors to improve shipping routes and cost savings. Includes, onboarding of new vendors.
Requirements:
- Bachelor’s Degree or 5+ years supply chain/logistics experience
- Minimum of 2+ years’ experience in Life science ,biopharmaceutical manufacturing or regulated supply chain activities preferred
- Must be self-motivating, organized and proactive
- Strong technical aptitude including demonstrated experience in applying scientific reasoning to solve complex problems.
- Ability to work hours necessary to support completion of work
- Computers and Electronics – Knowledge of electronic equipment, and computer hardware and software, including supply chain and inventory applications
1) BUSINESS DEVELOPMENT
- Attends to all inquiries.
- Does follow up of clients for the provided quotes
- Connects to new Customers.
- Prepares Business plan and Budget projection on year on basis.
2) MIS , REVIEW & MONITORING
- Updates open order sheet.
- Reviews MSL, Freight forwarder & Stock sheet
- Reviews Profit & Loss Sheet.
- Discusses everyday task with the HOD.
- Reviews the financial AR & AP for RLD business.
3) SOURCING
- Reviews & places PO to Suppliers.
- Takes follow up from suppliers from already placed PO.
- Connects to new Suppliers.
- Does follow up with suppliers and Ensures the readiness of the goods for the timely dispatch.
4) OPERATIONS ( LOGISTICS & DOCUMENTATION)
- Gives instructions to prepare shipping invoice & packing list to Documentation team & Logistics team.
- Plans shipment & share TO DO list to Logistics Team
- Shares priority list to USA warehouse team.
5) COMMERCIAL INPUTS
- Shares documents to Accounting Team.ie. Sales invoice, Purchase invoice, Swift copies.
- Solves query of Accounting Team.
6) TEAM MANAGEMENT
- Assign the task and Liase with internal logistic team , documentation team and reviewing their completion of task in correct and timely manner.
- Solves query of Logistics Team & Documentation Team.
- Reviews the Team work on weekly basis and take the corrective actions.
Qualification
- Pharmaceutical experience warehouse operation experience is must
- Good English verbal and written communications is must.
- Competent with MS office and computer basic skills.
- Punctual, Disciplined and planner of the day.
Job Description
- Receiving the goods into the goods checking the specification making entry into the Track n trace or into the Inventory management system.
- Taking 6 sided pictures and putting into the right storage racks as per the recommended temperature.
- Responsible for making the outbound shipment , packaging materials in correct boxes to fit the goods in.
- Handling the logistics part and coordinating with the logistics team and other procurement teams.
- Handling the single handedly the operation activity of the shipments coming in and going out.
- Pharmaceutical goods handling experience is preferable.
- Maintenance activity and coordinating with the tenant or lease owner.
- Responsible to secure and put the goods in secured conditions.
- Managing the physical stocks and accountability for the same coming in and going out.
- Expired goods, damaged goods to be putted in Quarantine area goods needs to be putted in different locations.
- Proper Quality control checks SOP needs to be followed.
- Temperature monitoring and making notifications in case of deviations.
- Strictly adhering and following the procedures of the warehouse operating systems.
- Ordering the packaging material with accountability at regular intervals.
- Monthly check of the inventory physical and stock in system of inventory of track and trace basically.
If you have the agility to adapt, adopt and elevatethe dynamism created by the young force at NewLife Medicals, we could be the next big contributor in ever evolving culture! If freedom to express, think, challenge, and innovate excites you, we could be the right blend in this culture. We have an open culture where the only hierarchy to follow is honesty and the only rules to follow are high productivity. You could be expanding our global territories, researching newer markets, challenging the market volatilities, strengthening our operations, spearheading our digital innovations, or managing our people. All we need from you is… you should be aspirational!
We offer a range of job as well as internship opportunities that will expose you to the real business environment and channelize your skills towards making a real difference in society.
Work with us
Opportunities based in India :
- Business Development – Key Accounts- Hyderabad
- Business Development – Exports (FF) - Ahmedabad
Opportunities based in USA & Ireland :
- Business Development – RLD - Raleigh , North Carolina
- Office Coordinator - Dublin , Ireland
Opportunities based in ROW:
Regional BD Representative in
- Far East Asia - South Korea
- Middle East Asia - Dubai
- South East Asia - Singapore
- Pacific - Australia
- Latam - Brazil
- Africa - Egypt
- Regional BD Representative - Far East Asia, South Korea
- Regional BD Representative - Middle East Asia, Dubai
- Regional BD Representative - South East Asia, Singapore
- Regional BD Representative - Pacific, Australia
- Regional BD Representative - Latam, Brazil
- Regional BD Representative - Africa, Egypt
Warehouse Executive:
Qualification
- Pharmaceutical experience warehouse operation experience is must
- Good English verbal and written communications is must.
- Competent with MS office and computer basic skills.
- Punctual, Disciplined and planner of the day.
Job Description:
- Receiving the goods into the goods checking the specification making entry into the Track n trace or into the Inventory management system.
- Taking 6 sided pictures and putting into the right storage racks as per the recommended temperature.
- Responsible for making the outbound shipment , packaging materials in correct boxes to fit the goods in.
- Handling the logistics part and coordinating with the logistics team and other procurement teams.
- Handling the single handedly the operation activity of the shipments coming in and going out.
- Pharmaceutical goods handling experience is preferable.
- Maintenance activity and coordinating with the tenant or lease owner.
- Responsible to secure and put the goods in secured conditions.
- Managing the physical stocks and accountability for the same coming in and going out.
- Expired goods, damaged goods to be putted in Quarantine area goods needs to be putted in different locations.
- Proper Quality control checks SOP needs to be followed.
- Temperature monitoring and making notifications in case of deviations.
- Strictly adhering and following the procedures of the warehouse operating systems.
- Ordering the packaging material with accountability at regular intervals.
- Monthly check of the inventory physical and stock in system of inventory of track and trace basically.



